Professor Tony Mok from the Faculty of Medicine of The Chinese University of Hong Kong (CU Medicine) and Professor Lu You from West China School of Medicine at The Sichuan University co-led the world’s first-in-human Phase I clinical trial investigating the safety and feasibility of CRISPR gene-edit therapy as a treatment option for patients with late stage lung cancer. Research team recruited 22 advanced Non-small-cell lung carcinoma (NSCLC) patients and isolate the T cell (a form of white blood cell) from peripheral blood. After gene-editing by CRISPR, the T cells that were reinfused back to patient may have the ability to attack cancer cell. Objective of the study is to demonstrate safety and feasibility. Results demonstrated CRISPR technology is safe and feasible as patients showed no severe adverse events and the frequency of off-target events was only 0.05%. This opens a new chapter in the history of lung cancer immunotherapy. The findings were recently published on-line in the international medical journal Nature Medicine.
Inhibiting PD-1 protein is the key to treat advanced NSCLC
Lung cancer is the most common cancer in the world, accounting for 1.6 million deaths every year. In Hong Kong, lung cancer is also the leading cause of cancer deaths and the second most common cancer with over 4,000 new cases every year. NSCLC accounts for about 80% of all lung cancers.

Professor Tony Mok Shu Kam, Li Shu Fan Medical Foundation Professor of Clinical Oncology and Chairman of the Department of Clinical Oncology said, “Anti-PD-1 checkpoint inhibitors are currently the standard first-line therapy for advanced NSCLC. PD-1 is a protein on the surface of our T cells in the immune system. When it binds with ligand PD-L1 from cancer cells, it prevents the immune system from killing cancer cells. The inhibitors serve the purpose to prevent the binding from taking place and allow the immune system to do its work and kill cancer cells. Based on the same theory, we are exploring the use of an alternative tool – CRISPR technology to edit T cell and disable PD-1 on T cell from functioning.”
World’s First-in-human Clinical Trial Using CRISPR Technology for Lung Cancer
Professor Lu You is the pioneer in clinical application of CRISPR technology in China, and in partnership with Professor Tony MOK who is also a visiting professor to West China School of Medicine, they designed the world’s first-in-human clinical trial with a team of scientists and oncologists from China. The research team recruited 22 advanced NSCLC patients who had failed multiple lines of therapy between 2016 and 2018 for the study. They implemented CRISPR-Cas9 technology to disrupt PD-1 gene in T cells. Total of 17 patients had sufficient T cells for the editing process but five dropped out because of disease progression and other reasons. Researchers extracted T cells from patients, edited them with CRISPR and re-infused as therapeutic T cells.
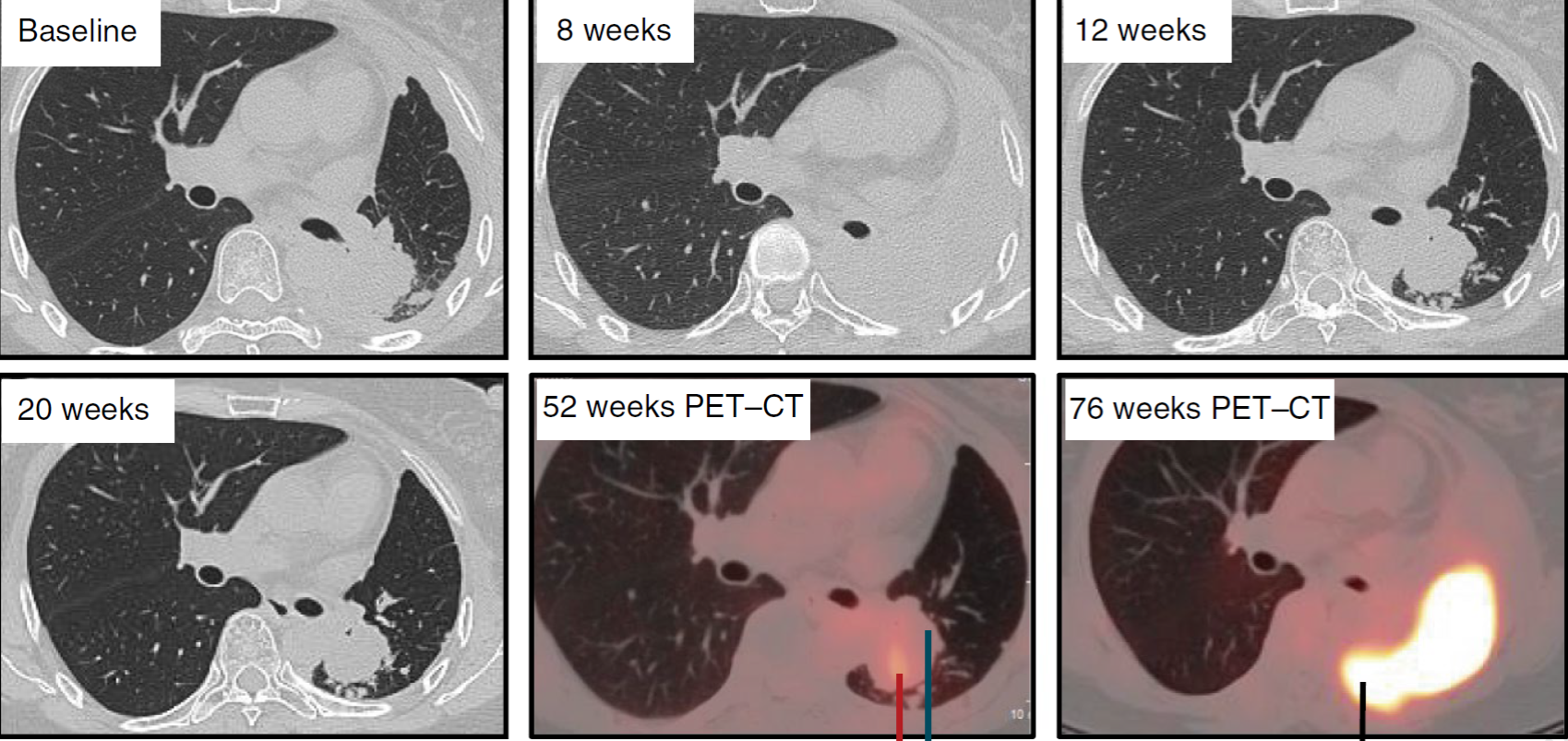
As per report in Nature Medicine, the median time to prepare the T cells for reinfusion for these 17 patients was 25 days. None of the 12 patients who received the treatment had severe or life-threatening adverse events. Given the risk of off-target mutations, which are the nonspecific and unintended genetic modifications that may potentially be harmful, whole-genome sequencing and next generation sequencing were performed on edited T cells before and after infusion. The median mutation frequency of off-target events was only 0.05%. The finding is strongly supportive of the fact that T cell gene-editing by CRISPR is safe.
Treatment efficacy was limited as this was not the objective of study and most patients were at very late stage of their illness. The best response was seen in a 55-year-old woman whose tumour mass initially shrank, and her disease remained stable for almost 75 weeks before finally progressing.
“To get a new technology or therapy into the clinic we first need to ask if it is feasible and if we can do it safely. A new chapter is opening in the cancer immunotherapy field, combining the cutting-edge technologies of CRISPR gene editing and T cell therapy and it is still in the early stage of development,” said Professor Tony Mok.
In regard to future improvement in efficacy, Professor Lu You commented, “Gene-edited cell therapy is definitely a potential new direction for treatment of lung cancer. An important point for future improvement gene editing therapy is to enrol patients earlier when the T cells are still in healthy state. At the end of the day, it is the T cell that does the work, and we need healthy T cells.”