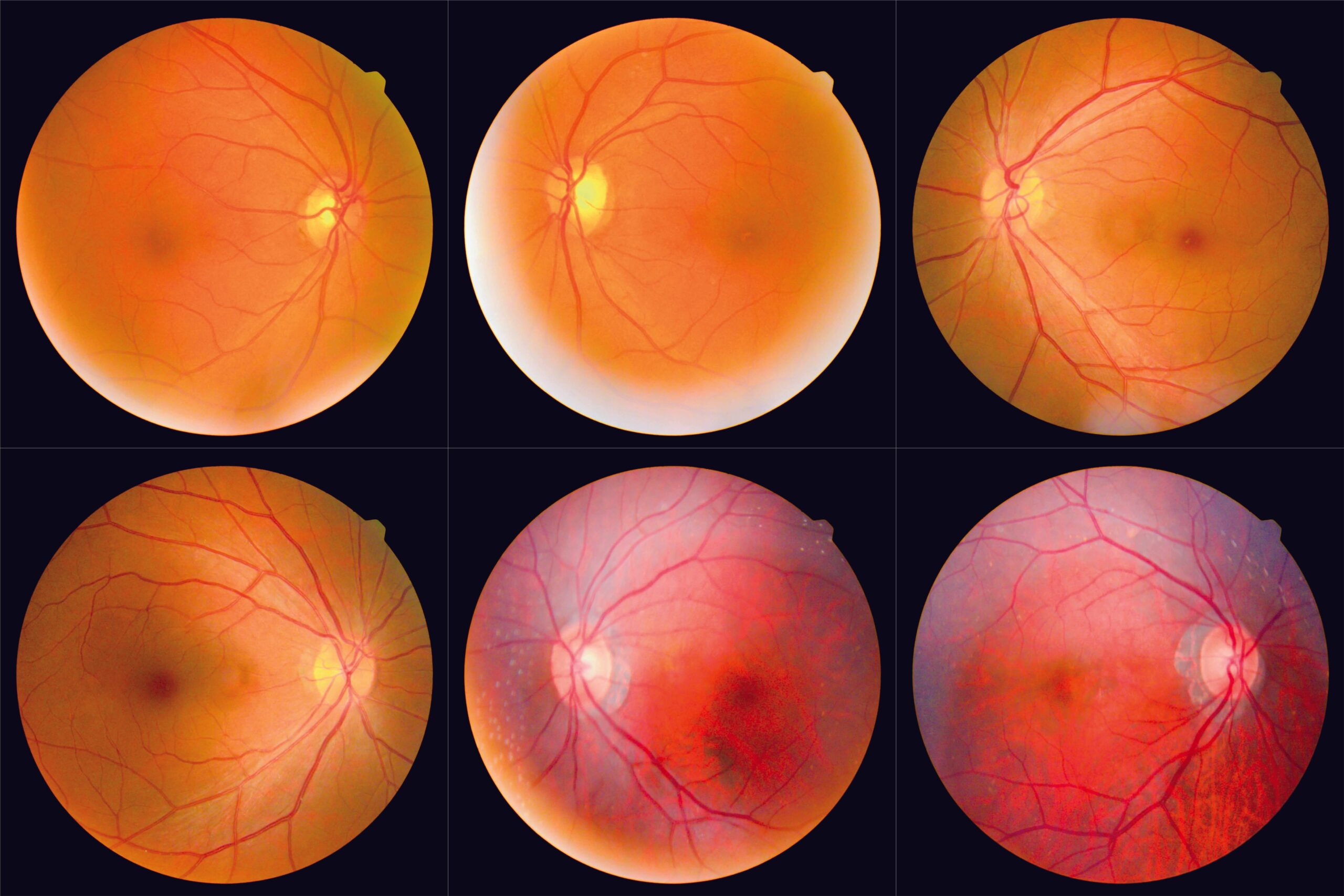
As societies across the world grow older, Alzheimer’s disease (AD) is a growing cause of suffering and distress, and a burden on health and social care systems. In Hong Kong, for example, about 10% of people aged 70 or above suffer from dementia, with about 60% of those cases attributable to Alzheimer’s disease.
Limitations of current diagnostic techniques
The disease is associated with an excessive accumulation of abnormal amyloid plaque and neurofibrillary tangles in the brain, causing brain tissue to shrink. The problem is, that can be difficult to detect. On MRI images, it isn’t obvious to the naked eye, and it shares symptoms with other common neurodegenerative diseases.
“Early detection of AD is complex, involving expensive and sometimes invasive investigations,” says Dr Carol Cheung, Associate Professor in the Department of Ophthalmology and Visual Sciences in the Faculty of Medicine of The Chinese University of Hong Kong (CU Medicine). “Currently, a definitive diagnosis of AD can only be made at the post-mortem. In clinical settings, the diagnosis of AD has been largely based on evaluation of symptoms and neurocognitive testing. However, using a syndrome to define AD is neither sensitive nor specific to the underlying neuropathologic changes of AD, and it cannot identify asymptomatic individuals who nonetheless have biological evidence of AD.”
Methods that can detect Alzheimer’s disease, such as an amyloid-PET scan or testing of cerebrospinal fluid collected via lumbar puncture, are invasive and inaccessible. As a result, Alzheimer’s patients often miss several years of preventive treatments, for example with anti-amyloid drugs, that can slow cognitive decline and brain damage.
A tool for early detection
An international team led by CU Medicine, with members from institutions in Singapore, the UK and the US, is trying to fix that. To do so, it’s looking to the fundus, the part of the eye opposite the retina. Part of the central nervous system, it’s already used to study diseases including diabetic retinopathy, age-related macular degeneration and glaucoma.

“Being an extension of the central nervous system, the retina shares physiological and anatomical similarities with the brain,” she says. “We just need to take fundus photographs for the diagnosis, which are an excellent window for direct, non-invasive evaluation of changes in the central nervous system related to AD.”
Taking fundus photographs is non-invasive, convenient and, as there’s no contact with the eye, pain-free. It’s also a fraction of the cost of an amyloid-PET scan; and the testing equipment is small, making it easy to set up at clinics. Patients just need to have a handful of photos taken, and the system CU Medicine team has developed can generate a risk report within minutes. It does so by using AI to crunch the visual data.

Artificial intelligence boosts the diagnostic accuracy
“Alterations in retinal vasculature and retinal neuronal structure may be associated with ageing and other ocular, neurological and systemic diseases. The strength of the associations between retinal imaging parameters and AD is relatively modest. Therefore, we need a more precise diagnostic method,” she says.
“Artificial intelligence has great potential to address these gaps. Deep learning technology is a major area of future research in the analysis of medical images to transform healthcare, focusing on image classification and pattern recognition. Deep learning permits algorithms to be trained through multilayer neural networks on big data.”
The team developed and validated the model using nearly 13,000 fundus photographs from 648 Alzheimer’s disease patients and 3,240 cognitively normal subjects. It showed 84% accuracy, 93% sensitivity and 82% specificity in detecting the disease. Across countries, its accuracy varied between 80% and 92%. As well as diagnostic tests in clinics, the system could also be used as a screening tool in the community.
Next, the CU Medicine team plans to develop an integrated, AI-based platform that can combine information from the blood vessels and nerves of the retina captured by fundus photography with optical coherence tomography, further boosting diagnostic accuracy. While the AI system is still in the early stages of development, the team aims to test it in combination with the fundus camera within a year, and hopes it will be available for clinical use within two. Further down the line, she says, the same techniques could potentially also be used to detect other conditions including glaucoma, diabetic macular edema and retinal diseases.